ABOUT
Freeox Biotech is an innovative late-stage clinical company singularly focused on the discovery and development of Cerebrovascular Protective Therapeutics. A Phase III-ready investigational drug – Ox-01, the company’s lead asset – has been shown to be an effective emergency adjunct therapy to Mechanical Thrombectomy for the treatment of Acute Ischemic Stroke (AIS). FreeOx’s leadership in this category was firmly established in early 2023, by the U.S. National Institute of Health/NINDS’ SPAN (Stroke Preclinical Assessment Network). As published in SCIENCE TRANSLATIONAL MEDICINE, Ox-01 (Uric Acid) demonstrated unprecedented efficacy compared to five other candidate interventions, and according to SPAN, the only drug candidate for which data supported further clinical evaluation.
STROKE OVERVIEW
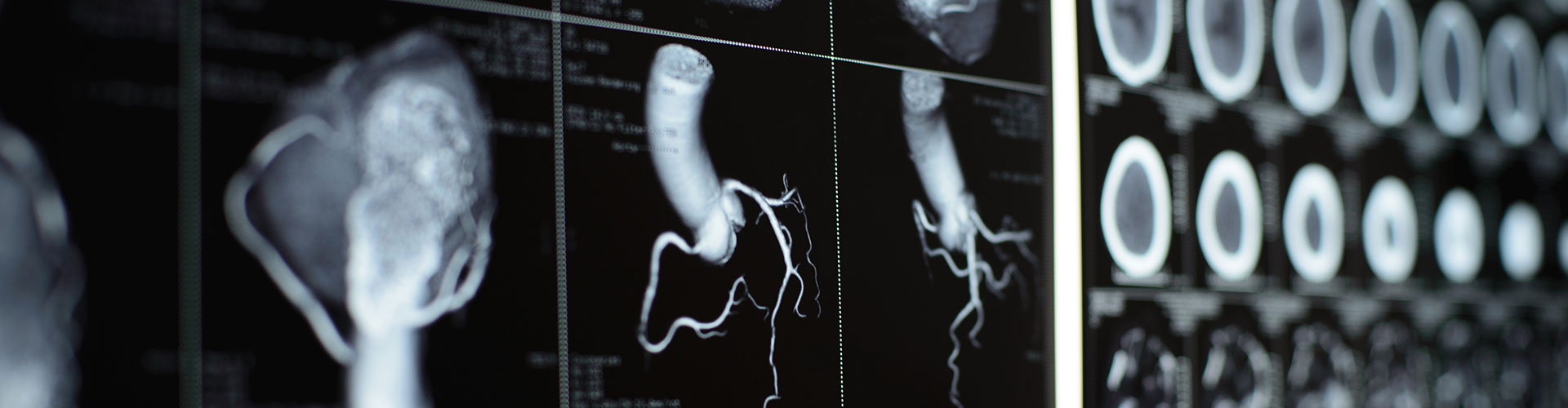
Stroke is a leading cause of death and disability worldwide. Up to 2 million brain cells die each minute the brain is deprived of oxygen hence, ‘Time is brain’ in stroke. Even with the best available treatments (rtPA – recombinant tissue Plasminogen Activator-, or MT-Mechanical Thrombectomy), only 50% of the 6 million patients who survive a stroke can live independently at 90 days post therapeutic intervention. The 3 million stroke survivors who cannot live independently ravage healthcare resources with indirect costs estimated for the US alone at more than $66 billion per year (2021). Ox-01, directly addresses this critical and long-standing unmet need.
By administering Ox-01 (active ingredient= pharmaceutical grade Uric Acid) during an emergency MT, we believe Ox-01 can enable functional independence for an additional 1 million AIS survivors worldwide every year. In 2015, the introduction of MT raised the standard of care for Large Vessel Occlusion (LVO) AIS patients. By recanalizing large vessels, MT improved outcomes for 50% of patients, compared to rtPA where only 30% of treated patients showed favorable results. Nevertheless, MT alone has its limitations as 50% of MT patients face the prospect of dependent living or worse. It is this very shortfall that was the focus of the NIH/NINDS (National Institute of Neurological Disorders and Stroke) SPAN Network randomized and double-blinded study reported in September 2023 in the peer-reviewed journal SCIENCE TRANSLATIONAL MEDICINE. Using a novel animal model to assess six MT adjunct treatment candidates for AIS, only Ox-01, Uric Acid, exceeded the trial’s final efficacy threshold and as such, in February 2023 was deemed by SPAN to be the only intervention that warranted further clinical evaluation. SPAN is considered to be the new benchmark in translational medicine for stroke, with other indications already following its footsteps.
Over 20 years of research underlies UA’s ground-breaking accomplishment reported by SPAN. Since our inception, we have sought to raise the standard of care for AIS patients and further improve the number of patients with good outcomes.
In the URICO-ICTUS Phase IIb trial, our founder — Dr. Ángel Chamorro — was the first to combine a reperfusion booster with Mechanical Thrombectomy, and first to demonstrate its safety. Central to our strategy is Uric Acid’s demonstrated ability to enhance microcirculation and boost penumbra reperfusion. Today, as we prepare for Phase III, FreeOx Biotech is the only company that has produced and developed Uric Acid as a pharmaceutical product (GMP) and administered it as an adjunct to MT in patients with AIS.

